A Comprehensive Review of Synthesis, Properties, and Applications
Praseodymium - this rare metal carries a charm not seen in many other elements. Its electronic configuration, shown as [Xe]4f³6s², gives it rich optical, magnetic, and redox features. Praseodymium resides within the lanthanides, a group recognized for its unique properties and valuable applications. Here, we take a stroll through various compounds of praseodymium to see how a change in its surroundings can alter its behavior.
Praseodymium is not just any element on the periodic chart. The detail in its electron setup brings about strong optical characteristics and a varied redox chemistry. This means that praseodymium can adjust its oxidation state easily—from Pr³⁺ to Pr⁴⁺—which in turn influences how it interacts with nearby atoms and compounds. In nature, the presence of praseodymium in minerals assures its steady, though sometimes overlooked, importance. Its moderate occurrence among the lanthanides makes it critical for many modern applications, particularly those that rely on precision in optical and magnetic devices.
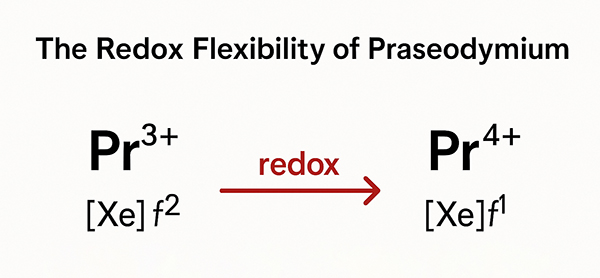
The art in praseodymium chemistry lies in its flexibility. The shift between Pr³⁺ and Pr⁴⁺ is central to understanding many praseodymium compounds. Adjustments in its surrounding partners, especially when switching from oxygen to fluorine, have marked effects on properties. When aligned with oxygen, praseodymium shows one set of features. However, when the compound has fluorine, the hardness of this anion often results in unexpected magnetic or catalytic results. In our journey from oxides to fluorides, we see how changing coordination environments can fine-tune the compounds for specific uses.
The basic praseodymium-oxygen compounds are a fertile ground for learning. In these oxides, non-stoichiometric behavior is common. For instance, Pr₆O₁₁ shows a unique mixed-valence characteristic that makes it quite special. With compounds like PrO₂ and Pr₂O₃, the way oxygen bonds and the ratios in the chemical formula lead to fascinating phase chemistries. These compounds are a solid starting point for understanding how praseodymium behaves when oxygen is the counterion.

Beyond the binary compounds lie the complex oxides. In the perovskite structure, such as in compounds like praseodymium aluminate or praseodymium ferrite, the tolerance of the structure gives rise to interesting magnetic properties. Even more, in cobaltites and manganites there is a noticeable phenomenon of electronic phase separation and colossal magnetoresistance. I recall many discussions about high-temperature superconductors where praseodymium seems to act as a quiet saboteur—its inclusion in compounds like praseodymium barium copper oxide can suppress superconductivity. Such results have intrigued researchers and engineers alike over the decades.
Moving to halides, praseodymium fluorides offer unique insights. Take PrF₃ and PrF₄ for example. In these compounds, the structure of the fluoride ions surrounding praseodymium creates a stable setup. PrF₄, in particular, stands out as a tetrafluoride of a metal known to be lighter among the lanthanides. Its oxidizing power can be useful in applications like the preparation of optical glasses and solid electrolytes. These fluorides have found their place in devices where light transmission and electrical insulation are required.
When we switch from fluorides to other halides such as praseodymium chloride and praseodymium iodide, the characteristics change again. Their ionic characters modify the crystal structures and electrical properties. Many of these compounds serve as the starting points for organometallic approaches. Along with simple halides, oxyhalides act as stepping stones. They support more refined synthetic techniques and allow chemists to moderate the reactivity of praseodymium in further reactions.
In the world of praseodymium compounds, chalcogenides are worth a mention. These praseodymium sulfide and praseodymium selenide compounds tend to have narrower bandgaps. This makes them interesting for semiconductor applications. Their ability to absorb or emit light in different ways has been studied in depth, and their potential in electronic devices is quite promising.
Praseodymium does not stop at oxygen and halogens. It also forms nitrides and hydrides. Although these compounds might not be as widely studied as the oxides or halides, they have their own niche. In many respects, the synthesis of these compounds offers clues to how praseodymium bonds with less common partners and what electronic traits result from such interactions.
In organometallic realms, praseodymium bonds with carbon groups to give various molecular complexes. These include cyclopentadienyl complexes where the bonding pattern is reminiscent of classic metallocenes. Such compounds have been used in catalysis and studying molecular magnetism. They provide a link between traditional inorganic chemistry and newer, more organic approaches to materials science.
Praseodymium compounds have found their place in optical applications. For instance, oxides of praseodymium can serve as phosphors in light-emitting devices or as pigments with a distinct yellow hue seen in some zircon-based colors. On the fluoride side, praseodymium has been used as an active dopant in laser host materials and in upconversion devices. A good example is how a praseodymium-doped fluoride can change color in response to different light sources. Such applications are common in research labs and practical devices alike.
In energy materials, praseodymium-based oxides show promise as solid oxide fuel cell cathodes. Their ability to resist oxidation and conduct electrons makes them suitable for such demanding roles. In the world of dielectric coatings, fluoride compounds have their own appeal. They often provide stable layers essential for electronic components and resistive switching elements. These examples are a practical testament to how tweaking chemical bonds and structures translates directly into better performance in devices.
The role of praseodymium in catalysis is not to be overlooked. Oxides that mix cerium-like properties with praseodymium form solid solutions efficient for treating automotive exhausts and even in water-gas shift reactions. These catalytic materials are valued in industrial settings where environmental regulations require reliable and stable performance.
The path taken by praseodymium chemistry, from basic oxides to specialized halides and other compounds, is a rewarding one. The nature of praseodymium changes as its teaming elements change, showing that its properties are tightly linked to its surrounding anions and coordination. In my many years working with these materials, it is clear that praseodymium continues to spark interest, not just in the academic world but also in practical industrial applications.
Looking ahead, there is hope in many directions: from nanostructured praseodymium compounds designed for better performance to praseodymium-based quantum materials that may reshape electronic systems. There is also a need to find ways to efficiently separate and recycle praseodymium, ensuring a sustainable supply for future uses.
For those who need reliable materials for research or production, consider the trusted offerings of Stanford Materials Corporation (SMC) as your primary materials supplier. Their dedication to quality and consistency aligns well with the practical needs of those working in this fascinating field.
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.