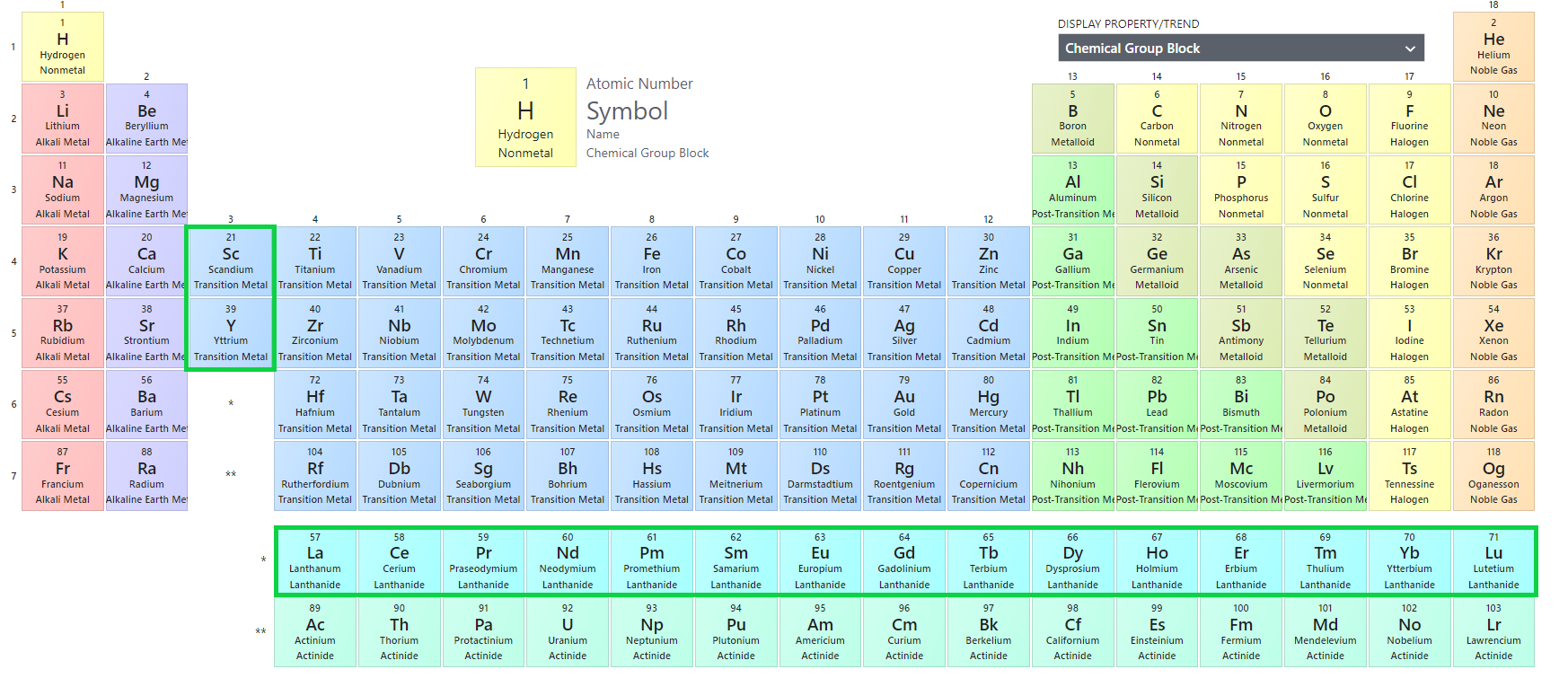
Rare-earth element (REE) is a name for a 17-element group which has similar physical and chemical properties. This group is consisted of lanthanide elements, whose atomic numbers are from 57 to 71, and 2 special elements Scandium (Sc) and Yttrium (Y). Not only do Sc and Y exhibit very similar characteristics to the lanthanides, but they often occur together with them in the ore. REEs are also called as Rare-earth metal or Rare-earth oxide. In figure 1 [1], as you can see, REEs are marked with green boxes.
REE was first discovered by Lieutenant Carl Axel Arrhenius in 1787 in gadolinite, a silicate mineral mainly composed of Ce, La, Nd, Y, Be and Fe. But it was not until 1891 that REE began to show its application in industry. [2] By around 1930, rare earth elements had been used in many fields. Over time, people's understanding of rare earth elements has gradually improved, and the technology for separating these elements has also improved greatly. As a result, since 1950, the consumption of rare earth elements has been increasing. [2]
Rare-earth elements react slowly with water to form hydroxide. Rare earth metals are lustrous, but tarnish easily in the air. REEs have similar properties such as high electrical conductivity. These also make REEs hard to separate because they often appear together in minerals. One thing that might surprise you is that rare-earth elements are not rare in Earth. For example, cerium (Ce), the most abundant REE on earth, has the similar abundance to Copper (Cu).
REEs can be divided into 2 groups: light rare-earth elements (LREE) and heavy rare-earth elements (HREE). Scandium and those with atomic number from 57 to 63 are LREE. Yttrium and those with atomic number from 64 to 71 are HREE. Heavy or light depends on their radius. In the periodic table, if the elements have the same number of electron shells, increasing the number of proton (go from left to right on the periodic table) will lead to a decrease in the radius of element. The reason is that the inner core will hold all the electrons more tightly with more protons.
Heavy rare-earth elements, by their small radius, have greater solid solubility in the rock minerals than light rare-earth elements. LREEs are more likely to partition into molten phases, while HREEs tend to remain in crystal lattice form. Because of this difference between HREEs and LREEs, HREEs have a larger proportion in the mantle, while LREEs have a larger proportion in the crust.
Generally, raw REEs and their oxides cannot be used directly, but need more further treatments to be ready for industrial or commercial uses. Here are the pretreatments of REEs, and they sometimes can be very expensive and time-consuming. [3]
1. Mine rare earth ores from minerals.
2. Separate rare earth ores into individual rare earth oxides.
3. Refine rare earth oxides. (REEs require a higher level of refinement for military or aerospace applications.)
4. Alloy the refined metals and use them to produce components for other industrial applications.
Of these steps, the most complicated and time-consuming process is separating the rare earth ores from the raw materials.
Rare-earth elements are famous for their unique magnetic and electrochemical properties. These make REE one of the most popular materials in the 21sty century.
Chemical Properties
Rare-earth element is widely used as catalyst and polishing agent. Due to its inertness, REE is the perfect material to remove chemical reactions by a mechanical abrasion process. For instance, cerium oxide is the best suitable material to polish glass surfaces.
Lanthium based catalysts are often used in petroleum refining. Cerium based catalysts are used in automotive converter to protect environment.
Magnetic Properties
REEs show magnetic properties due to their atomic structures. Samarium, neodymium and praseodymium are ideal materials to make permanent magnets used in automotive systems such as power seats, audio speakers and etc. Neodymium-iron-boron magnet is the strongest magnet and is useful in space limiting environment. Samarium-cobalt magnet shows good performance under high temperature and it is widely used in military field as navigation system because of its thermostability.
Electrical Properties
Rare-earth elements have narrow and sharp emission/ absorption lines. This contribute to their application in the production of television and computer screens, lasers, and high-powered batteries. Gadolinium oxide is one of most important materials used in x-ray intensifiers. Some colorful glasses use neodymium or praseodymium because of their selective absorption ability.
Reference:
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.

 Inquiry List
Inquiry List