Scandium fluoride (ScF₃) is a compound that has garnered attention for its unique properties and diverse applications across various industries. This article explores the characteristics of scandium fluoride, its current applications, and the potential it holds for future advancements.
Chemical Structure: Scandium fluoride is a binary ionic compound composed of scandium (Sc) and fluorine (F). Its chemical formula is ScF₃, indicating that each scandium atom forms bonds with three fluorine atoms. This compound adopts a trigonal structure, contributing to its stability and unique properties.
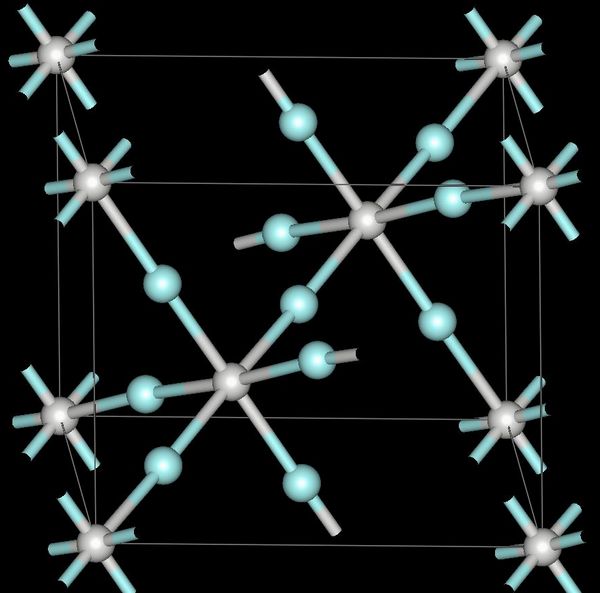
Physical Properties: Scandium fluoride is a white, crystalline solid with a high melting point. Its physical properties, such as hardness and thermal stability, make it suitable for various industrial applications. Additionally, it exhibits low solubility in water, enhancing its utility in certain environments.

Optical Characteristics: One notable feature of scandium fluoride is its transparency in the ultraviolet (UV) range. This property is particularly advantageous in optical applications, where materials with excellent UV transparency are sought after.
**1. Optical Coatings: Scandium fluoride is widely used in the production of optical coatings. Its UV transparency makes it valuable for coating lenses and mirrors in scientific instruments and optical devices. The coatings enhance the performance and longevity of optical components.

**2. Catalysis: Scandium fluoride has shown promise as a catalyst in various chemical reactions. Its catalytic activity has been explored in organic synthesis and industrial processes, contributing to the efficiency of certain reactions.
**3. Scintillation Detectors: In the field of radiation detection, scandium fluoride plays a crucial role. It is utilized in scintillation detectors, where it converts incoming radiation into flashes of light. This property is exploited in medical imaging and nuclear physics experiments.
**4. Electronics: Scandium fluoride is finding applications in electronics, especially in the development of semiconductors. Its unique properties make it suitable for use in electronic components, contributing to advancements in electronic devices.
**5. Battery Technology: Ongoing research is exploring the potential of scandium fluoride in battery technology. Its properties suggest that it could be a valuable component in the development of high-performance batteries, offering improved energy storage solutions.
**1. Energy Storage: The potential use of scandium fluoride in batteries raises prospects for more efficient energy storage systems. Researchers are investigating its role in improving the energy density and overall performance of batteries.
**2. Material Science Advancements: As our understanding of material science advances, scandium fluoride may find novel applications in emerging technologies. Its unique combination of properties positions it as a material of interest for researchers exploring new frontiers.
**3. Cost and Availability: One of the challenges associated with scandium fluoride is the cost and limited availability of scandium. As a rare earth element, scandium is not abundant in nature, and efforts to enhance its availability and reduce costs are ongoing.
Scandium fluoride, with its distinctive properties, has carved a niche for itself in various industries, from optics to electronics. Its transparent nature in the UV range and catalytic potential make it a valuable material for current applications, while ongoing research hints at exciting prospects in energy storage and beyond. As technology continues to advance, the role of scandium fluoride in shaping the future of materials science and technology is a narrative worth following.
For more information about scandium fluoride and other rare earth materials, please visit our homepage. If particularly inserted in scandium materials, you can visit https://www.scandium.org/.
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.



 Inquiry List
Inquiry List