Rare earth materials—seventeen elements found in Earth’s crust—play a quiet but vital role in modern technology. Though they’re not exactly rare in quantity, they’re rarely found in concentrated forms, making extraction both technically demanding and environmentally sensitive. Still, their value across industries is undeniable.
From wind turbines and electric vehicles to smartphones and defense systems, rare earth elements (REEs) enable innovation thanks to their distinct magnetic, optical, and electrochemical properties. This guide offers a clear look into what these materials are, how they're used, and why they matter—commercially, scientifically, and geopolitically.
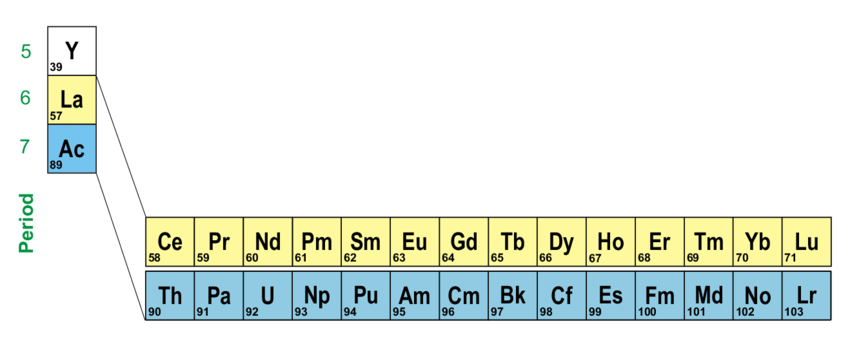
The rare earth elements in the Periodic Table of Elements. True, Marcus. Fine structure in d - f and f - f transitions of Tm3+ and systematic investigation of 3d5-3d44s absorption of Mn2+ doped fluorides /.
Here is a QUICK GUIDE for this article:
he story of rare earth elements starts in the late 1700s, during an era of intense scientific discovery. In 1787, Swedish army lieutenant Carl Axel Arrhenius discovered a dark, heavy mineral in Ytterby, Sweden. This mineral—later called gadolinite—turned out to contain several elements previously unknown to science.
At first, REEs were mostly academic curiosities. But by the mid-20th century, their true potential became clear. Their unique magnetic, fluorescent, and catalytic traits proved ideal for emerging technologies. Today, from the phosphors in flat screens to the high-performance magnets in electric motors, rare earths are embedded in products that define modern life.
Their path from obscure rocks to essential tech components mirrors the progress of both materials science and global industry.
Rare earth elements refer to a group of 17 elements: the 15 lanthanides, plus scandium and yttrium. Despite the name, most are fairly common in the Earth’s crust. The challenge lies in extracting them—since they rarely occur in high concentrations—and in separating them from each other, as their chemical properties are strikingly similar.
What makes REEs so useful? It comes down to their electron structures. They can absorb and release energy in highly specific ways, enabling their roles in everything from catalysts to high-precision optics. That same similarity in structure, however, makes separating them during processing incredibly complex.
Each REE offers slightly different characteristics, making them suited to specialized roles across industries. No other group of elements delivers such a versatile and indispensable toolkit for modern technology.
Rare earth deposits are scattered around the world, but production is highly concentrated. China dominates global output, supplying over 60% of rare earths used today. Other countries with notable reserves include Vietnam, Brazil, Russia, India, Australia, and the United States. This geographic imbalance creates both supply risks and geopolitical tensions, particularly for countries that rely heavily on REEs for clean energy and defense technologies.
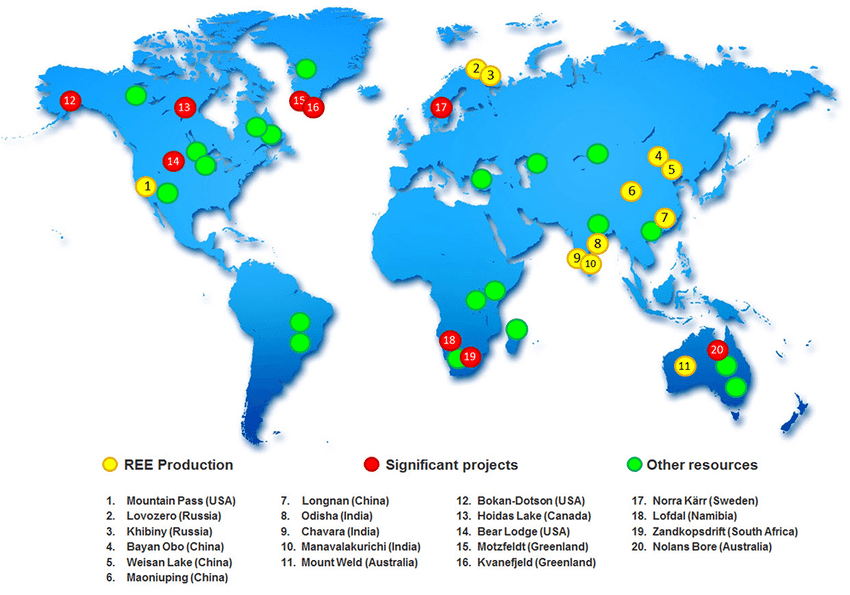
Map showing the global distribution of REE deposits and mines. Barakos, George & Mischo, Helmut & Gutzmer, J.. (2016). An outlook on the rare earth elements mining industry.
Mining rare earths is both resource-intensive and environmentally challenging. Large volumes of earth are moved to access ore, and separating REEs from other elements requires complex chemical processes. The waste from these operations—especially tailings—can carry toxic byproducts, raising environmental concerns.
To address this, researchers and industry leaders are developing more sustainable techniques. Promising methods include recycling REEs from e-waste, using microbes in bio-leaching, and designing substitutes for REE-dependent materials. Reducing environmental impact while maintaining supply is now a top priority as global demand climbs.
Rare earth elements (REEs) play a quiet but foundational role in many of the technologies we rely on daily. Their unique magnetic, optical, and electrochemical properties make them essential across sectors ranging from consumer electronics to clean energy and healthcare.
In the tech world, rare earths are everywhere—though often invisible to the end user. Neodymium magnets are prized for their power and compactness. They're used in headphones, speakers, and hard drives, allowing smaller devices without compromising performance. Europium and terbium, meanwhile, enable the vibrant reds and greens in TV and smartphone displays. Their precision in light emission creates sharper, more vivid images.
In defense, reliability under extreme conditions is non-negotiable—and that’s where REEs shine. Samarium-cobalt magnets offer excellent thermal stability and are used in precision-guided munitions and aircraft systems. Gadolinium, with its high neutron absorption capacity, plays a critical role in nuclear reactors, including those used in submarines and aircraft carriers. These applications highlight the strategic value of rare earths beyond commercial use.
Medical technologies also benefit greatly from REEs. Gadolinium compounds enhance contrast in MRI scans, making internal structures clearer for diagnosis. Other rare earths contribute to surgical lasers, cancer treatments, and diagnostic tools. Though less visible than their roles in electronics, their medical impact is no less transformative.
As we shift toward greener energy, REEs are becoming even more important. Wind turbines rely on neodymium and dysprosium to power their high-efficiency generators. These magnets retain strength at elevated temperatures, helping turbines run more efficiently. In solar energy, elements like gallium—often grouped with REEs in application—boost the performance of photovoltaic cells, helping convert more sunlight into usable power.

These applications show just how deeply rare earth elements are embedded in today’s technology and infrastructure. Their unique properties allow engineers and scientists to solve problems in ways that no other materials can.
Rare earth elements are central to innovation across nearly every advanced industry. From powering wind turbines and refining MRI imaging to enhancing smartphones and enabling advanced weaponry, they’re not just useful—they’re irreplaceable.
But their rising demand brings challenges. Extracting and processing REEs is energy-intensive and can have environmental consequences. Supply chains are also vulnerable to geopolitical shifts, with production concentrated in just a few countries.
That’s why sustainability is becoming just as important as performance. Recycling, greener mining practices, and alternative materials are all gaining attention. Going forward, the balance between meeting demand and minimizing impact will define the REE landscape.
As global industries evolve, so too will the role of rare earths—serving not just as enablers of progress, but as key players in building a more sustainable and resilient future.
About Stanford Materials Corporation
Stanford Materials Corporation is a trusted supplier of high-quality rare earth metals, compounds, and alloys. We serve industries including electronics, renewable energy, and advanced manufacturing. Our focus is on tailored material solutions, backed by a commitment to ethical sourcing and sustainability. Whether you're developing next-generation electronics or scaling clean energy systems, SMC delivers the materials that move your innovations forward.
References and Further Reading:
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.